To calculate the mass of BeI₂, we first need to convert it to number of moles using the Avogadro's number:
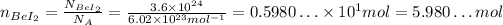
Now we need to calculate the molar mass of BeI₂ using the molar masses of the atoms Be and I, which we can consult on a periodic table:

And now we can calculate the mass:
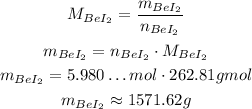
Which corresponds to alternative A.