Answer: Option (c) is the correct answer.
Step-by-step explanation:
An equation will be balanced when both number of reactants and products are equal.
The given equation is as follows.
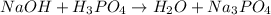
Number of reactants are as follows.
Number of reactants are as follows.
To balance the equation, multiply NaOH on the reactant side by 3 and
 on the product side by 3.
on the product side by 3.
Therefore, the equation will be as follows.
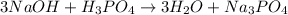
The coefficient in front of NaOH is 3.