To solve this problem, we're going to use the formula of molarity:
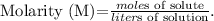
We have to find the number of moles in 106 g of Na. The molar mass of Na can be found in the periodic table, which is 23 g/mol. The conversion would be:
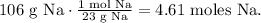
Finally, we replace the moles (4.61 moles) and the liters of solution (6.00 L) in the formula:
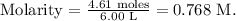
The molarity of the solution would be 0.768 M.