Answer: The pressure of an enclosed gas will depend on the number of molecules in a unit volume and and their average kinetic energy.
Explanation: The pressure of the gas is inversely related to volume given by Boyle's Law.

And pressure is directly related to the temperature of the gas given by Gay-Lussac's Law

Relation between average kinetic energy and temperature is:
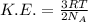
From this relation, we can say that pressure is related to the average kinetic energy.
From the above relations, we can say that the pressure depends on the number of molecules in a unit volume and and their average kinetic energy.