Answer:
The number of photons are
 .
.
Step-by-step explanation:

where,
E = energy of photon =
h = Planck's constant =

c = speed of light =
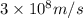
 = wavelength = 10.0 μm =
= wavelength = 10.0 μm =

1 μm =



Let the n number of photons with energy equal to E' = 1.0 kJ = 1000 J



The number of photons are
 .
.