Answer: The volume occupied at STP is 1.345 L
Step-by-step explanation:
The equation given by ideal gas follows:
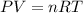 .......(1)
.......(1)
where, P = pressure of the gas
V = Volume of the gas
T = Temperature of the gas
R = Gas constant =
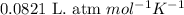
n = number of moles of gas
We are given:
![P=0.15atm\\V=2.5L\\T=15^oC=[15+273]=288K\\n=?](https://img.qammunity.org/2017/formulas/chemistry/high-school/qec87dl0y8ieitar0qwqcpc6g246nbxo7h.png)
Putting values in equation 1, we get:
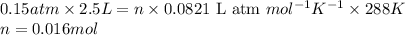
We are given:
![P=0.42atm\\V=2.5L\\T=15^oC=[15+273]=288K\\n=?](https://img.qammunity.org/2017/formulas/chemistry/high-school/8hw2lqz60qg1xhft6u88nkagtfij8o3y5x.png)
Putting values in equation 1, we get:
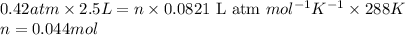
- Now, calculating the volume occupied by helium and neon at STP when nitrogen gas is removed by using equation 1, we get:
STP conditions:
T = 273 K
P = 1 atm
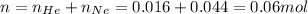
Putting values in equation 1, we get:
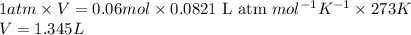
Hence, the volume occupied at STP is 1.345 L