ANSWER
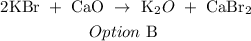
Step-by-step explanation
Given that;
The two reactants are KBr and CaO
Double replacement reaction is a type of chemical reaction that occur when two reactants exchange cations and anions to yield new products.
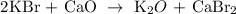
Therefore, the resulting products of the given data are K2O + CaBr2
The correct answer is option B