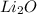Answer: The chemical formula for the compound of lithium and oxygen will be
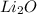
Step-by-step explanation:
Ionic bond is defined as the bond which is formed when complete transfer of electrons takes place from one atom to another atom.
Lithium is the 3rd element of the periodic table with electronic configuration of

This element looses 1 electron and shows an oxidation state of +1.
Oxygen is the 8th element of the periodic table with electronic configuration

This element will gain 2 electrons and shows an oxidation state of -2.
By criss-cross method, the oxidation state of oxygen becomes the coefficient of lithium and that of lithium becomes of oxygen.
Hence, the chemical formula for the compound obtained by the combination of lithium and oxygen is