If 4.5 g of Hydrogen is to be formed then this mass would have in x mol
x mol = Mass of H
÷ Molar Mass of H
= 4.5g ÷ ( 1 x 2) g/mol
= 2.25 mol
mole ratio of CaH₂ : H₂
1 : 2
∴ if mol of H₂ = 2.25 mol
then mol of CaH₂ = 2.25 mol ×
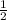
= 1.125 mol
Mass of CaH₂ = 1.125 mol × [(40 × 1) + (2 × 1)]
= 47.25 g