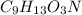Answer : The empirical formula of a compound is,
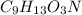
Solution : Given,
If percentage are given then we are taking total mass is 100 grams.
So, the mass of each element is equal to the percentage given.
Mass of C = 59 g
Mass of H = 7.1 g
Mass of O = 26.2 g
Mass of N = 7.7 g
Molar mass of C = 12 g/mole
Molar mass of H = 1 g/mole
Molar mass of O = 16 g/mole
Molar mass of N = 14 g/mole
Step 1 : convert given masses into moles.
Moles of C =
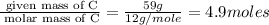
Moles of H =
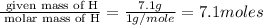
Moles of O =
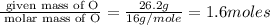
Moles of N =
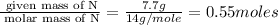
Step 2 : For the mole ratio, divide each value of moles by the smallest number of moles calculated.
For C =
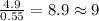
For H =
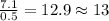
For O =
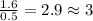
For N =
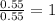
The ratio of C : H : O : N = 9 : 13 : 3 : 1
The mole ratio of the element is represented by subscripts in empirical formula.
The Empirical formula =
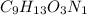 =
=
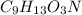
Therefore, the empirical formula of a compound is,