Answer:Mass NaN3 = 222.73 grams
Explanations:
GIVEN:
• mass of N2 =, 144 g
,
• Molecular Mass N2 = ,28.02 g/mol
,
• Molecular Mass NaN3 = ,65,0099 g/mol
The balanced equation of the chemical reaction is given by :
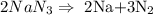
(i) Calculate moles of N2
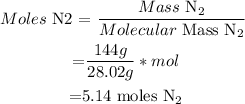
This means that 5.14 moles are produced from NaN3
(ii) Determine moles and mass of NaN3 needed to produce 144 grams of N2.
by stoichiometry, we can see that :
• 2 Moles of NaN3 produces 3 moles N2
,
• X moles of NaN3 produces 5.14 moles N2
Therefore;
X Moles NaN3 =( 5.14 moles N2 *2 moles NaN3 )/ 3 moles N2
=3.43 moles of NaN3
Then ; Mass of NaN3 will be calculated as :
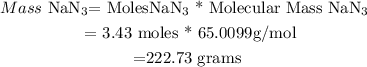
This means that Mass of NaN3 needed to produce 144g of N2 = 222.73 grams