They give us the volume of the substance, to calculate the mass we can relate the property of density which is equal to:
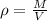
Where,
M is the mass of the substance
V is the volume
The density of Fe2O3 can be found in the table of properties of substances. At standard conditions, the density is equal to: 5.242 g/cm3.
The mass will be:
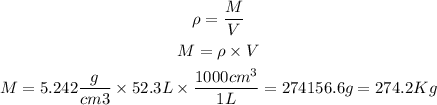
So, the mass of 52.3L of Fe2O3 is 274.2 Kg