Answer:
% Free space in water =
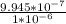 ×100 = 99.45%
×100 = 99.45%
% Free space in ice =
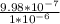 ×100 = 99.8%
×100 = 99.8%
Step-by-step explanation:
As given ,
Density for ice at 0⁰C = 0.917 g/ml
Density for water at 0⁰C = 0.999 g/ml
Radii of H atoms = 37 pm
Radii of O atoms = 66 pm
Now,
Consider 1 ml of water = 1 cm²
As , we know that mass of water in 1 cm² = 0.999 g
Moles of water =
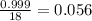
Volume of H₂O = 1.624×
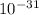 m²
m²
Now,
Volume occupied by water = 0.056×6.022×
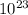 × 1.624×
× 1.624×
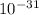 m²
m²
= 5.48×
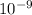 m²
m²
⇒Volume occupied by water = 5.48×
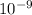 m²
m²
Now,
Free space = 1×
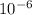 - 5.48×
- 5.48×
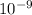 = 9.95×
= 9.95×
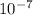 m²
m²
% Free space =
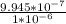 ×100 = 99.45%
×100 = 99.45%
Now,
Consider 1 ml of ice = 1 cm²
S.I unit of ice = 1×
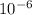 m²
m²
As , we know that mass of water in 1×
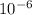 m² = 0.917 g
m² = 0.917 g
Moles of ice =
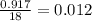
Volume of H₂O = 6.022×
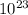 ×0.012
×0.012
Volume of ice unit =
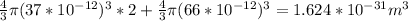
Now,
Volume occupied by water = 0.012×6.022×
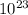 × 1.624×
× 1.624×
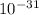 m²
m²
= 1.17×
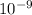 m²
m²
⇒Volume occupied by water = 1.17×
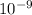 m²
m²
Now,
Free space = 1×
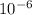 - 1.17×
- 1.17×
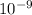 = 9.98×
= 9.98×
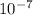 m²
m²
% Free space =
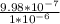 ×100 = 99.8%
×100 = 99.8%