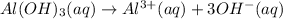Step-by-step explanation:
Bases are those substance which releases hydroxide ions in aqueous solution when dissolved in water.

As we can see that in formula of aluminium hydroxide , there are 3 hydroxide ions attached to single aluminum ion. So, when we will dissolve the aluminum hydroxide in water it will give aluminum ions and hydroxide ions in its solution.