The answer is a) 7 half-lives and b) 0.78% (or as fraction 1/128) of the starting amount.
a) If half-life of Polonium-210 is 138 days, to calculate how many half-lives occur in 966 days, we will simply divide them: 966/138 = 7
So, 7 half-lives occur in 966 days.
b) To calculate the remaining amount, we will use the formula:

where n is the number of half-lives, and x is the remaining amount in decimals, and (1/2) is half-life.
We've already found that n = 7, so replace it in the formula:
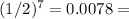
0.78% = 1/128