Answer: Option (C) is the correct answer.
Step-by-step explanation:
It is known that oxidation number of sodium is +1 and the oxidation number of oxygen is -2.
Let the oxidation number of tin (Sn) is x.
Therefore, calculate the oxidation number of tin in the given formula as follows.
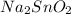
= (2 \times oxidation number of Na) + Oxidation number of Sn + (2 \times oxidation number of O)
=

So, 2 + x - 4 = 0
therefore, x = +2
Thus, we can conclude that the oxidation number of tin (Sn) in the compound
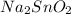 is +2.
is +2.