Answer: the volume of the metal is 5.02 mL
Step-by-step explanation:
The question requires us to calculate the volume of a sample which density is 92 g/mL and mass is 462 g.
Measurements > Density
The density of a substance is defined as the ratio between its mass and volume:
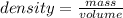
We can rearrange the equation above to calculate the volume of a sample from its density and mass:
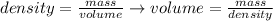
And, applying the values of mass and density given by the question, we'll have:
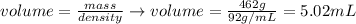
Therefore, the volume of the metal is 5.02 mL.