Answer:
a. AgCl.
b. AB.
c. 1.3 moles AgCl.
d. The net ionic equation is:
![Ag^++Cl^-\operatorname{\rightarrow}AgCl.]()
Step-by-step explanation:
a. first, let's remember the concept of a precipitation: precipitation is the process of conversion of a solution into solid by converting the substance into insoluble form or by making the solution a super saturated one. You can see that the precipitate in this case, would be AgCl because this compound is not soluble in water.
b. You can see that the chemical equation is already balanced because we have the same number of elements on both sides. AB.
c. You can see in the chemical equation that 1 mol of AgNO3 reacted, produces 1 mol of AgCl, so the molar ratio between them is 1:1, so 1.3 moles of AgNO3 reacted produces 1.3 moles of AgCl. You can see better this by the following conversion:
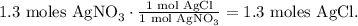
d. To write the net ionic equation, we have to 'separate' the ions in each compound. For example, we have Ag (+), NO3 (-), K (+) and Cl (-) ions, like this:
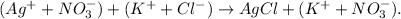
And then, we 'cancel' the terms we have the same on both sides, in this case, K(+) and NO3 (-) to obtain our net ionic equation:
![Ag^++Cl^-\operatorname{\rightarrow}AgCl.]()