Answer:
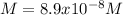
Step-by-step explanation:
Hello,
In this case, one can assume 1L as the volume of the solution, so we've got 0.010mg of cadmium. Now, as we're asked to know its molarity, one computes the moles of cadmium as follows:
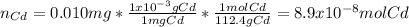
Now, one obtains the molar concentration (molarity) as shown below:
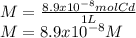
Best regards.