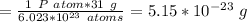Answer:
Mass of Avogadro's number of P atoms = 31 g
Mass of 1 P atom = 5.15*10⁻²³ g
Step-by-step explanation:
The two rules to consider are:
1) Mass of 1 mole of an element is equal to its molar mass in grams
2) 1 mole of any element contains Avogadro's number of atoms
i.e. 1 mole = 6.023*10^23 atoms(or molecules or particles)
For P-31
1 mole of P-31 = 31 grams
i.e. Mass of Avogadro's number of P atoms = 31 g
6.023*10^23 P atoms = 31 g
Therefore, mass of 1 P atom is: