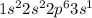Step-by-step explanation:
According to Aufbau's rule, in the ground state of an element, electrons start to fill atomic orbitals from the lowest energy level before they occupy higher energy levels.
Electronic configuration is the distribution of electrons in the atomic orbitals of an atom.
Atomic number of sodium is 11 and its electronic configuration is as follows.