Answer:
Volume of a gas is directly proportional to its temperature and inversely proportional to its volume.
Step-by-step explanation:
As per the gas law
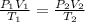
where
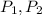 are pressure of gas
are pressure of gas
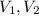 are volume of gas
are volume of gas
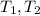 are temperature of gas
are temperature of gas
This equation shows that the pressure is inversely proportional to the volume of gas and directly proportional to the temperature of a gas
Thus, if pressure remains constant, the volume of gas will also remain constant (if all other physical and chemical condition remains the same)