You first need to find the molar mass of ethane to figure this out.
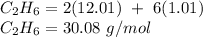
You then need to find the mass of carbon by multiplying its molar mass by how many molecules of carbon there are in the compound.
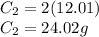
You then simply divide the mass of carbon by the mass of the entire compound.

Multiply this value by 100 to get your percentage.
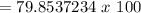
= 79.85% (round as you wish)