Answer:
C2H2
Step-by-step explanation:
Mass of Carbon in CO2
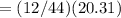 grams
grams
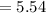 gram
gram
Mass of hydrogen in H2O
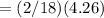 grams
grams
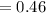 gram
gram
We will calculate the % of C and H in the sample
% C
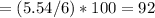 %
%
% H
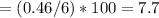 %
%
Number of mole of C
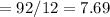
Number of mole of H
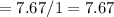
Fractional share of C
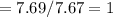
Fractional share of H
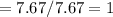
Empirical Formula = CH
Empirical Mass
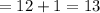
Molecular mass
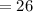
Molecular Formula – CHn
N
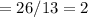
Molecular Formula = C2H2