Answer:
The radius of the ball is 3.48 cm
Explanation:
Density
The density of a substance is the mass per unit volume. The density varies with temperature and pressure.
The formula to calculate the density of a substance of mass (m) and volume (V) is:
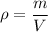
The density of lead is \rho=11.35\ g/cm^3 and the mass of a ball of lead is m=2 kg=2,000 g. We can calculate the volume by solving for V:
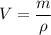
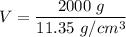
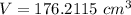
Since the shape of the object is a sphere, and the volume of a sphere of radius r is:
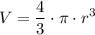
We can solve for r and obtain:
![\displaystyle r=\sqrt[3]{(3V)/(4\pi)}](https://img.qammunity.org/2022/formulas/mathematics/high-school/ikkl3yw6kb609xlgov67kt2tzlqr80vai4.png)
Substituting:
![\displaystyle r=\sqrt[3]{(3*176.2115)/(4\pi)}](https://img.qammunity.org/2022/formulas/mathematics/high-school/ah7fnj015k29ycmjbbrqf2eyqkhz4lkluk.png)
![\displaystyle r=\sqrt[3]{42.0674}](https://img.qammunity.org/2022/formulas/mathematics/high-school/m4fqg2t4mqukgtlw5mxcb54k3wzu78dhv7.png)
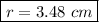
The radius of the ball is 3.48 cm