We are asked to calculate the number of ions of lead in paint.
Given that paint has:
Volume = 3.00 L
density = 4.65 g/mL
The mass of Lead (II) nitride in paint is = 33.1 g
Firstly let us write the equation:
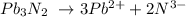
We can calculate the number of moles of Pb3N2 and use stoichiometry to find the number of moles of Pb.
number of moles (n) of Pb3N2:
n = m/M where m is the mass and M is the molar mass of Pb3N2
n = 33.1g/649.61 g/mol
n = 0.0509 moles
number of moles of Pb:
Using the stoichiometry, the molar ratio between Pb3N2 and Pb is 1:3 according to the equation.
Therefore number of moles of Pb is 0.0509 moles x 3 = 0.1529 moles.
After calculating the number of moles, the number of ions will be equal to the product of the number of moles and Avogadro's number.
number of Pb ions = number of moles x avogadros number
number of Pb ions = 0.1529 moles x 6.022x10^23
number of Pb ions = 9.2076x10^22