Answer:
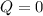
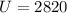
- Energy increases
Step-by-step explanation:
From the question we are told that
Work done
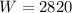
a)Generally the heat flow for an adiabatic process is 0 (zero)
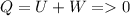
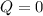
b)Generally Change in internal energy of gas is mathematically given by
Since
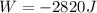
Therefore
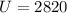
Giving
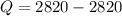
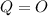
c)With increases in internal energy brings increase in temperature
Therefore
Energy increases