Answer:
Option B - 15299
Explanation:
Given : A fossilized leaf contains 15% of its normal amount of carbon 14. Use 5600 years as the half-life of carbon 14.
To find : How old is the fossil (to the nearest year)?
Solution :
Let the normal or initial amount of carbon be A,
According to the question,
An exponential function form
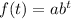
Where, a is the initial i.e, a=A
A fossilized leaf contains 15% of its normal amount of carbon 14.
So, f(t)=15% of A
i.e,
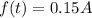
b is half-life of Carbon-14 b=0.5
and t is the time span or age of the fossil in years.
Substitute all the values in the formula,
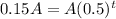
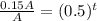
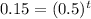
Taking log both side,
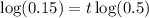
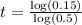
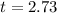
For 5600 years the age of the fossil is
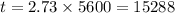
Therefore, Approximately Option B is correct.
The fossils are 15299 years old.