Step-by-step explanation:
A solution is defined as the substance formed due to addition of two or more number of substances. A solution is a homogeneous mixture.
And, homogeneous mixtures are always clear solution. Particles of a homogeneous solution never combine chemically with each other.
For example, when we dissolve baking soda in water then it will completely dissolve in water forming a clear solution.
So, in order to determine that it is a solution we can let the water from the solution to evaporate.
This will leave behind the solute, that is, baking soda in the vessel. This will show that it is a solution.
Whereas a compound is defined as the substance in which different elements are chemically combined together in a fixed ratio by mass.
For example,
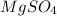 is a compound and elements are present in 1:4 ratio.
is a compound and elements are present in 1:4 ratio.
Components of a compound can only be separated by chemical means and not by any physical means.