Answer: Option (B) is the correct answer.
Step-by-step explanation:
Reactants are the species which are present on the left hand side of a chemical equation.
On the other hand, products are the species which are present on the right hand side of a chemical equation.
For example,
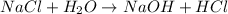
Here, NaCl and H_{2}O are the reactants whereas NaOH and HCl are the products.
This also means that products are the new substances which are formed.
Thus, we can conclude that new substances that are present at the end of a reaction accurately describes the products of a reaction.