Answer:
4C₃H₃N + 3O₂ → 12C + 6H₂O + 2N₂
Step-by-step explanation:
The given reaction equation is expressed as:
C₃H₃N + O₂ → C + H₂O + N₂
The problem here entails balancing of the chemical reaction.
So;
assign letters a,b,c,d and e as coefficients that will balance the expression:
aC₃H₃N + bO₂ → cC + dH₂O + eN₂
Conserving C : 3a = c
H : 3a = 2d
N : a = 2e
O: 2b = d
let a = 1, c = 3, e =
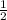 , d =
, d =
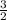 , b =
, b =
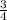
Multiply through by 4;
a = 4, b = 3, c = 12 , d = 6 and e = 2
4C₃H₃N + 3O₂ → 12C + 6H₂O + 2N₂