Answer:
the difference in hydrogen ion concentration between these two solution is :
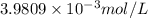
Step-by-step explanation:
Concentration of hydrogen ions when pH of the solution is 11.2(alkaline)
![pH=-\log[H^+]](https://img.qammunity.org/2017/formulas/chemistry/high-school/2ja0ild124jpqk99oa1zclrbpxonz2t1fz.png)
![11.2=-\log[H^+]](https://img.qammunity.org/2017/formulas/chemistry/high-school/r10umodcb2hqh31xsotf0wi08iqjab9yke.png)
![[H^+]=6.3095* 10^(-12) mol/L](https://img.qammunity.org/2017/formulas/chemistry/high-school/3ffz7q91r9xgthxr2x5xfveawcj2yanxx1.png)
Concentration of hydrogen ions when pH of the solution is 2.4 (acidic).
![pH=-\log[H^+]'](https://img.qammunity.org/2017/formulas/chemistry/high-school/swpdq6nnss7a7ndsq8ftd2qvpd7a0gogpt.png)
![2.4=-\log[H^+]'](https://img.qammunity.org/2017/formulas/chemistry/high-school/9cyrzzchqbayij12nsrxqbnh5m7g6y080x.png)
![[H^+]'=0.39810 mol/L](https://img.qammunity.org/2017/formulas/chemistry/high-school/mp5qi5fycq5ejdalslrzl76tlmec414wv2.png)
In an acidic solution hydrogen ion s concentration always larger than that of of the alkaline solution.
![[H^+]'>[H^+]](https://img.qammunity.org/2017/formulas/chemistry/high-school/zna9jh6u2cgqffu5fhpa1dfji7av2ex90k.png)
The approximate difference in the concentration of hydrogen ions between the two solutions:
![[H^+]'-[H^+]=0.39810 mol/L-6.3095* 10^(-12) mol/L=0.0039809 mol/L=3.9809* 10^(-3) mol/L](https://img.qammunity.org/2017/formulas/chemistry/high-school/lhp59272e91xmuuj9rtsejrpkv04f34oem.png)