Answer: The molar mass of the element is 40 g/mol and element is Calcium.
Step-by-step explanation:
At STP:
1 mole of a gas contains 22.4 L of volume
Molar mass is defined as the mass of 1 mole of a substance.
To calculate mass of of the substance, we use the equation:

Volume of gas = 22.4 L
Density of gas = 1.7824 g/L
Putting values in above equation, we get:
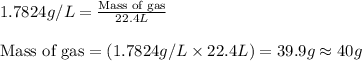
The element having molar mass 40 g is Calcium.
Hence, the molar mass of the element is 40 g/mol and element is Calcium.