Answer: The correct answer is Option c.
Step-by-step explanation:
The equation for the combined ideal gas law is:
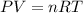
This law has arrived from the combination of four laws:
1) Boyle's Law: This law states that pressure is inversely proportional to the volume of the gas at constant temperature.
 (At constant temperature)
(At constant temperature)
2) Charles' Law: This law states that volume is directly proportional to the temperature of the gas at constant pressure.
 (At constant pressure)
(At constant pressure)
3) Gay-Lussac's Law: This law states that pressure is directly proportional to the temperature of the gas at constant volume.
 (At constant volume)
(At constant volume)
4) Avogadro's Law: This law states that volume is directly proportional to the number of moles of the gas at constant pressure and temperature.
 (At constant temperature and pressure)
(At constant temperature and pressure)
From the above information, we get that the correct answer is Option c.