Answer:
Option A. Pressure increases.
Explanation:
As per Boyle's Law of gases
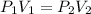
It means multiplication pressure and volume always remains constant.
If we increase the pressure, volume will decrease and when we increase the volume pressure decreases.
In other words pressure and volume are inversaly proportional.
Therefore, if volume decreases in a gas then pressure increases.