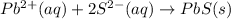Answer:
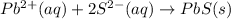
Step-by-step explanation:
Spectator ions are defined as the ions which does not get involved in a chemical equation or they are ions which are found on both the sides of the chemical reaction present in ionic form.
The given chemical equation is:
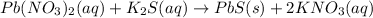
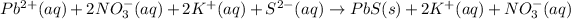
The ions which are present on both the sides of the equation are potassium and nitrate ions and hence are not involved in net ionic equation is:
Hence, the net ionic equation is