Answer:
KClO is an ionic compound
Step-by-step explanation:
Compounds can be broadly classified as ionic and molecular (covalent).
Ionic compounds are compounds in which the atoms are linked by ionic bonds formed by the transfer of electrons between them. These are formed between a metal and non-metal.
Molecular compounds are compounds in which the atoms are linked by covalent bonds formed by the sharing of electrons between them. These are formed between two non-metals.
In the given compound,
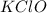 (potassium hyperchlorite), the potassium (K) atom is an alkali metal whereas the ClO moiety is composed of non-metals chlorine (Cl) and oxygen (O). Hence KClO is an ionic compound which exists as: K⁺ and ClO⁻
(potassium hyperchlorite), the potassium (K) atom is an alkali metal whereas the ClO moiety is composed of non-metals chlorine (Cl) and oxygen (O). Hence KClO is an ionic compound which exists as: K⁺ and ClO⁻