Answer:
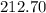 grams
grams
Step-by-step explanation:
Weight of one mole of Cl2 in grams =
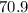
Weight of one mole of PCl3 in grams =
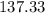
Weight of one mole of chlorine atom in grams =
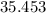
From one molecule of PCl3, three molecule of chlorine gas can be produced.
Weight of three molecule of chlorine gas
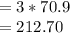 grams
grams