Answer:
n=5
Step-by-step explanation:
We use the Rydberg formula to solve this problem:
1 / λ =
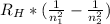
Where λ is the wavelength of absorption/emission, RH is a constant (1.097*10⁷m⁻¹), n₁ is the state of minor energy and n₂ is the state with higher energy.
- First, for the description for the absorption we have λ=92.3 * 10⁻⁹m, and n₁ = 1, so we solve for n₂:
1 / 92.3 * 10⁻⁹m = 1.097*10⁷m⁻¹ *
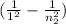
0.988 =
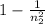
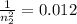
n_{2}^{2}=83.333\\\_{2}=9.12[/tex]
So after absorbing the wavelength of 92.30 nm the state of the hydrogen atom is n=9
- Now for the emission, we have λ=1820 *10⁻⁹m = 1.82*10⁻⁶m, and n₂=9, so we solve for n₁:
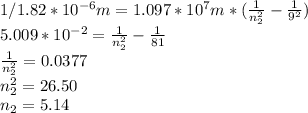
So the final state of the hydrogen atom is n=5