Answer:
See the figure 1.
Step-by-step explanation:
For the acetate ion we will have a formula of
 . We have to keep in mind that we will have 1 electrons for each hydrogen, 4 electrons for each carbon and 6 electrons for each oxygen and 1 for negative charge.
. We have to keep in mind that we will have 1 electrons for each hydrogen, 4 electrons for each carbon and 6 electrons for each oxygen and 1 for negative charge.
So, we will have in total:
H=>(1x3)= 3 e-
C=>(2x4)= 8 e-
O=>(2x6)= 12 e-
-1=> 1 e-
Total= 3e- + 8e- + 12e- + 1e- = 24e- (Figure 2)
When we do the formal charge for each atom we will have:

H=> FC=1-(0+
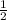 *2)=zero
*2)=zero
C=> FC=4-(0+
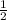 *8)=zero
*8)=zero
O=>FC=6-(4+
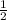 *4)=zero
*4)=zero
O=>FC=6-(6+
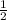 *2)=-1
*2)=-1
The total charge of the molecule is -1.