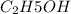Answer:
1. C. 18 atoms of carbon
2. C. Air
Step-by-step explanation:
1. The formula of one molecule of glucose is

In this formula we have:
Carbon: 6 atoms
Hydrogen: 12 atoms
Oxygen: 6 atoms
if we have three glucose molecules, we have to multiply the formula (
 ) by three
) by three
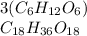
In this formula we have:
Carbon: 18 atoms
Hydrogen: 36 atoms
Oxygen: 18 atoms
2. A homogeneous mixture is the combination of 2 or more unidentifiable elements or substances within the solution.
Homogeneous mixtures are characterized by being uniform, this means that the elements that compose it are not distinguishable.
A. Oxygen is molecule

B. Water is molecule compose by 2 elements

C. Air is a mixture of several gases.
D. Alcohol is compound of carbon, hydrogen and oxygen.