Answer: The total number of particles present in the sample are
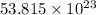 particles.
particles.
Step-by-step explanation:
We are given a total mass of the sample 103.75 grams in which 30% are helium atoms and 70% are krypton atoms.
The mass of 30% of helium atoms in the given amount of sample is =
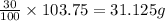
the mass of 7% of krypton atoms in the given amount of sample is =
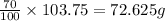
According to mole concept:
1 mole of an element contains
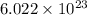 atoms/particles.
atoms/particles.
1 mole of helium contains 4 grams.
4 grams of helium element contain
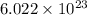 atoms/particles.
atoms/particles.
So, 31.125 g of helium element contain
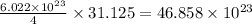 atoms/particles.
atoms/particles.
1 mole of krypton contains 84 grams.
84 grams of krypton element contain
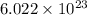 atoms/particles.
atoms/particles.
So, 72.625 g of krypton element contain
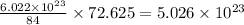 atoms/particles.
atoms/particles.
Total number of particles in the given sample =
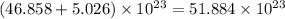 atoms/particles.
atoms/particles.