Answer:
37.4 mol.
Step-by-step explanation:
Hello!
In this case, since the Avogadro's number help us to realize that one mole of any substance contains 6.022x10²³ formula units, in this case atoms of zinc, the following dimensional analysis provides the correct answer:
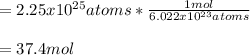
Best regards!