Answer: The correct answer is Option C.
Step-by-step explanation:
We are given:
Molarity of isoamyl salicylate = 0.75 M
This means that 0.75 moles of isoamyl salicylate is present in 1 L or 1000 mL of solution.
- To calculate the mass of isoamyl salicylate, we use the equation:
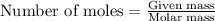
Moles of isoamyl salicylate = 0.75 moles
Molar mass of isoamyl salicylate = 208.25 g/mol
Putting values in above equation, we get:

By applying unitary method, we get:
156.19 grams of isoamyl salicylate is present in 1000 mL of solution.
So, 117.2 grams of isoamyl salicylate will be present in =
 of solution.
of solution.
Thus, 117.2 grams of isoamyl salicylate is present in 750 mL of solution.
- To calculate the mass of solution, we use the equation:

Density of solution = 0.7893 g/mL
Volume of solution = 1000 mL
Putting values in above equation, we get:

By applying unitary method, we get:
156.19 grams of isoamyl salicylate is present in 789.3 g of solution.
So, 117.2 grams of isoamyl salicylate will be present in =
 of solution.
of solution.
Thus, 117.2 grams of isoamyl salicylate is present in 592 g of solution.
Hence, the correct answer is Option C.