In order to calculate the amount of heat required, let's use the following formula:
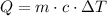
Where Q is the energy, m is the mass, c is the specific heat and DeltaT is the difference in temperature.
In this question, let's use m = 56.8 g, c = 4.186 J/g°C and DeltaT = 13.6 °C (that is, from 22.2 °C to 35.8 °C), so we have:
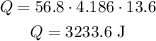
Therefore the amount of energy required is 3233.6 Joules.