Answer:
The mass of the liquid = 162 g
Step-by-step explanation:
As we know ,
Q = mc (ΔT)
where Q = Heat energy of the liquid
c = specific heat of the liquid
ΔT = change in temperature
m = mass of the liquid
As given , the temperature increases from 20.°C to 33°C,
⇒ ΔT = 33 - 20 = 13°C
Also given , c = 0.57 cal/g°C
Q = 1200 cal
∴ we get
1200 = (m)(0.57)(13)
⇒ 1200 = m(7.41)
⇒m =
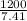 = 161.943 ≈ 162 g
= 161.943 ≈ 162 g
So, we get
The mass of the liquid = 162 g