Hello!
How many molecules are there in 15.7 liters of chlorine gas at STP?
We have the following information:
Knowing that for each mole of substance we have 6.02 * 10²³ molecules, it is known that in STP (Standard Temperature and Pressure) one mole of any gas equals 22.4 L, then:
22.4 L ----------------- 6.02*10²³ molecules
15.7 L ----------------- y molecules
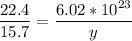
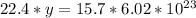
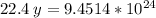

________________________
I Hope this helps, greetings ... Dexteright02! =)