Answer: the best option to answer the question is letter c, "Zinc is the limiting reagent"
Step-by-step explanation:
The question requires us to analyze the chemical reaction and amount of reactants given and choose the best statement to describe the reaction, considering limiting and excess reactants.
Stoichiometry > Limiting reactant > Determining the limiting reactant
The balanced chemical equation given is:
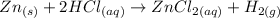
According to the balanced chemical equation, 1 mol of zinc (Zn) is necessary to completely react with 2 moles of hydrochloric acid (HCl). Therefore, the stoichiometric proportion given by the reaction is 1 mol of Zn and 2 moles of HCl.
Next, we must determine the limiting reactant when 1.5 moles of Zn and 3.4 moles of HCl are used. Note that these amounts do not correspond to the stoichiometric proportion.
If 2 moles of HCl are necessary to react with 1 mol of Zn, we can calculate how many moles of HCl would be necessary to react with 1.5 moles of Zn and compare to the stoichiometric proportion:
1 mol Zn ---------------- 2 mol HCl
1.5 mol Zn ------------- x
Solving for x, we have:
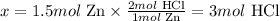
Therefore, 3 moles of HCl would be necessary to completely react with 1.5 moles of Zn.
We can also calculate the amount of Zn necessary to completely react with 3.4 moles of HCl:
2 mol HCl ------------ 1 mol Zn
3.4 mol HCl --------- y
Solving for y, we have:
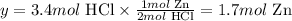
Therefore, 1.7 moles of Zn would be necessary to completely react with 3.4 moles of HCl.
Considering the amount of Zn and HCl given by the question, we can say that:
- 3 moles of HCl would be necessary to completely react with 1.5 moles of Zn. Note that the amount of HCl given is more than the amount necessary (3.4 moles given, 3.0 moles necessary), thus we can say that HCl is the excess reactant.
- 1.7 moles of Zn would be necessary to completely react with 3.4 moles of HCl. Note that the amount of Zn given is less than the amount necessary (1.5 moles given, 1.7 moles necessary), thus we can say that Zn is the limiting reactant.
Given the considerations above, the best option to answer the question is letter c, "Zinc is the limiting reagent".