They give us the mass of H2O, to determine the moles we will use the molar mass of H2O equal to 18g/mol
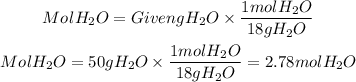
a. The number of moles of H2O is 2.78
Now, the number of molecules is found using Avogadro's relation, which tells us that in one mole of any substance, there will be 6.022x10^23 molecules. So the molecules of H2O will be:
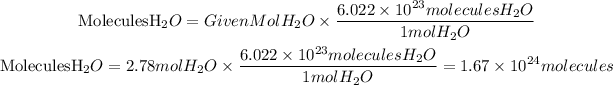
b. There are 1.67x10^24 molecules in 50 grams of H2O