Answer:
The length of the copper wire is 50.02 meters.
Step-by-step explanation:
It is given that,
Density of the copper wire,
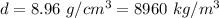
Diameter of copper wire, d = 0.25 mm = 0.00025 m
Radius of the copper wire, r = 0.000125 m
Mass of the copper, m = 22 g = 0.022 kg
We need to find the length of the wire. Let l is the length of the wire. The density of a copper wire is given by :
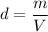
V is the volume of copper wire

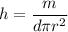
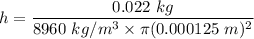
h = 50.02 m
So, the length of the wire is 50.02 meters. Hence, this is the required solution.